Making and Testing a Battery Smaller than a Grain of Pollen
With the introduction of electric charging with in-situ microscopy scientists finally have the tools to study the nanoscale charging/discharging reactions that make batteries work. Producing a battery this small isn’t easy, and the preparation techniques can introduce contamination that spoil the results. Researchers at the Karlsruhe Institute of Technology (KIT) and the Joint Research Laboratory Nanomaterials (KIT and TUD) in Germany have uncovered several sample preparation techniques that greatly improve accuracy and reliability of in-situ results.

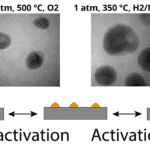
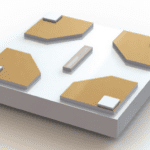
Instead of making a traditional Li-ion battery, which is susceptible to electron beam damage of the liquid electrolyte when imaging in TEM, the researchers chose to study all-solid-state fluoride-ion batteries. The ball-milled powder for the anode (Mg,MgF2, C, and La0.9Ba0.1F2.9 mixture), cathode (Cu and C composite), and electrolyte (La0.9Ba0.1F2.9) of the full cell were layered in a die, and compressed to 5Gpa to minimize porosity and ensure electrical contact between neighboring grains. A 30 um x 60 um section, 8 um thick was then removed from the consolidated pellet using focused ion beam (FIB) liftout and secured with Pt contacts using Electron Beam Induced Deposition (EBID) on the SiN membrane of the E-Chip. EBID was found to reduce the inherent contamination of Pt deposition by 3-10x compared to conventional ion beam deposition, which can short circuit the battery if not mitigated. The battery was then biased from 0 to 180 mV in the TEM, where only 0.5-1.5nA of additional current was induced from the electron beam.
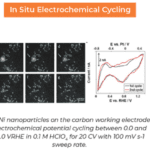
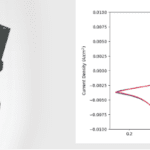
Additionally, a half-cell battery (Bi, C and La0.9Ba0.1F2.9 cathode, and La0.9Ba0.1F2.9 electrolyte) was made using the same technique and charged from 0 to 3V over 1 hour. The simultaneous acquisition of the I-V charging curve, image, and diffraction data showed the formation of BiF3 at the cathode and a corresponding reduction of La0.9Ba0.1F2.9 in the electrolyte.
View the full article and results in the recent publication: www.onlinelibrary.wiley.com.