Introduction
Cryo-electron microscopy (cryo-EM) is a form of transmission electron microscopy (TEM) based on the principle of cryogenically freezing biological samples in order to study them at high resolution under a TEM. Developed in 1990, this technique has yielded unprecedented insight into the molecular structure of biological samples, a development so profound that the pioneers of Cryo-EM, Jacques Dubochet, Joachim Frank and Richard Henderson were awarded the Nobel Prize for Chemistry in 2017.
This technique was developed because biological and organic samples typically exhibit poor contrast in the TEM and decompose when exposed to high-energy electrons. In addition, traditional organic sample preparation for TEM often severely damaged the biological structures, so new techniques needed to be created in order to image these materials. Cryo-EM also allows researchers to analyze large, complex, and flexible structures, create high-resolution images, and create precise, 3D reconstructions of the isolated molecule they are studying.
While incredibly useful for groundbreaking biological research, cryo-EM does come with its own set of challenges. Let’s take a look at a few:
Common Cryo-EM Research Challenges
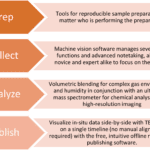
The biggest challenge with cryo-EM is sample preparation, primarily because it is extremely time-consuming and often prone to error; a single sample can take up to a month of preparation before it is ready for viewing. This is mainly due the iterative process of determining the best protocol for selecting, isolating, staining, dispensing, blotting, and freezing the sample. These factors all affect the final image quality and can destroy biological samples along the way.
One of the most challenging steps in preparing samples is the freezing process, also called “plunging”, where the sample is rapidly quenched to temperatures below -160ºC, causing the water to freeze so suddenly that it adopts an amorphous state, called “vitrified” ice which is necessary for imaging. However, the plunge freezing process can often cause samples to rupture, so the procedure must be refined until the sample survives the process.
Another challenge is finding an appropriate carbon support grid for placing cryogenically frozen samples on to. Since the thickness of the vitrified ice should be consistent for all samples imaged, TEM grids with carbon films that are more flat typically produce better ice uniformity and image clarity. Additionally, the thickness of the carbon film must be thick enough to support heavy samples, but thin enough to maximize image quality. It is recommended that the mesh size and carbon thickness of the holey carbon TEM grids be optimized for each sample studied in the lab.
The Necessity of Holey Carbon Grids for Cryo-EM
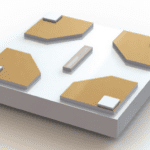
Holey carbon TEM grids are the sample support used for cryo-EM samples. The sample material is dispensed on the grid and rapidly quenched in liquid ethane near liquid nitrogen temperature. There are many reasons why using a holey carbon grid is necessary for cryo-EM research, including:
- Pristine Flatness: Holey carbon grids are designed to be as flat as possible. The reason for this flatness is thin and even ice distribution for optimal viewing quality. The pristine flatness of a holey carbon grid allows researchers to achieve superior cryo-EM data collection.
- Variety: Holey carbon grids vary in hole diameter, mesh size, film thicknesses, and mesh material to accommodate numerous types of research needs.
- Compatibility: Holey carbon grids are typically designed to be compatible with automated data collection software, which results in more high-quality target sites per grid.
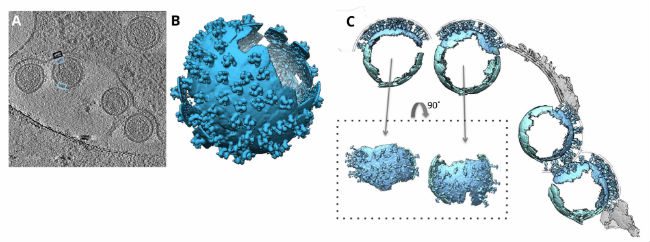
Holey Carbon Grid Use Case: Cryo-EM of Retroviral Envelope
In 2016, researchers from the University of Oxford and the University of Vienna used C-Flat holey carbon grids to support their samples of retroviral murine leukemia cells. After dispensing their sample, blotting it dry, and plunge freezing, the researchers were able to acquire cryo-EM images for tomograms, or two-dimensional image slices through a three-dimensional object. The tomograms were then used to reveal the underlying chemical structures of the murine leukemia cells.
The tomograms showed “massive structural rearrangements” of the murine leukemia virus envelope protein while the virus was attacking host cells. Researchers compared virus-free cells with virally-attacked cells and saw that the viruses lacked a clearly discernable bi-layer at the surface of the plasma membrane. This notable bi-layer structure difference implied that lipid rearrangement or mechanical stress could be influential factors in the leukemia virus attack.
Final Thoughts
Cryo-EM research techniques are becoming more and more popular, and will inevitably become increasingly common considering its recent attention for being awarded the 2017 Nobel Prize in Chemistry. Cryo-EM pioneers Dubochet, Frank, and Henderson’s work across the past three decades has filled the technology void in biochemical imaging. As a result of their cryo-EM innovations, researchers can now routinely produce 3D structures of biomolecules at near-atomic resolution, offering new insight on the molecular structures underpinning biological reactions.