2.5 million.
That’s how many phones Samsung had to recall after realizing the lithium-ion batteries used in the phone were prone to spontaneous combustion. Although some have speculated shoddy construction was to blame, Samsung was only one of the dozens of companies forced to recall these types of batteries in the past five years due to overheating or fires.
Clearly, there is a problem with lithium-ion batteries.
Why Are Lithium-Ion Batteries Prone to Explosion?


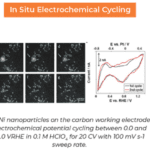
Lithium electrode growth while charging in a new dilute electrolyte (left). (Right) while charging, lithium deposits on the electrode, causing accumulation of large, crystalline dendrites that nearly completely dissolve on uncharging.
Just like all batteries, when lithium-ion batteries are charged and uncharged, the electrodes transfer electrons back and forth through the liquid electrolyte via traditional electrochemistry reactions. Unlike other batteries however, this electrochemical reaction is not fully reversible, leading “dead” lithium dendrites on both the electrode and floating in the electrolyte. If enough of this metal accumulates, the battery could short circuit, causing electrical failure in the best case, or combustion in the worst case. Despite decades of research on improving the efficiency and safety of lithium-ion batteries, the buildup of lithium dendrites appeared to be inevitable.
Monumental Lithium-Ion Battery Research Using In Situ Electrochemistry STEM
But researchers at Pacific Northwest National Lab may have discovered the solution to inhibiting the accumulation of this dead lithium.
In work recently published in Nature Communications, Dr. Layla Mehdi and colleagues used in situ liquid electrochemistry STEM to study the deposition and stripping of lithium in a nanoscale battery as it was charged and uncharged. They used the electrochemistry capabilities of their Poseidon Select system, which allows direct electrical measurements to be taken within the TEM.
Their insight came while they were watching lithium dendrites grow in different concentrations of electrolyte. They discovered that by adding only 50 ppm of water to the electrolyte, the lithium deposition during charging went from small, spongy particles to large, single lithium grains. As a result, the larger grains more energetically favored complete dissolution during uncharging, causing far less dead lithium to accumulate. This finding solves one of the key problems inhibiting the efficiency of lithium-ion batteries and could pave the way for a new generation of high powered batteries.
Final Thoughts
Dr. Mehdi is no stranger to the field of battery research, having published dozens of papers in top journals since finishing her PhD in 2012. Her work on the “Quantification of Electrochemical Nanoscale Processes in Lithium Batteries by Operando ec-(S)TEM” won her the distinguished 2015 M&M Post-Doctoral Researcher Award from the Microscopy Society of America.
Dr. Mehdi presented much of her electrochemistry work in a webinar hosted by Protochips and Materials Today. Entitled “In Situ TEM – the New Frontier for Liquid Chemistry”, the webinar featured research in the field of functional nanomaterials.
Learn more about the wide range of battery research capabilities, made possible by in situ microscopy.